
Irritable Bowel Syndrome (IBS) is a common chronic functional bowel disease with prevalence rates from 5 to 22%. IBS is defined by the coexistence of abdominal pain or discomfort and alteration in bowel habits without obvious organic abnormalities. Various treatments exist in this field, but what is the real current situation?
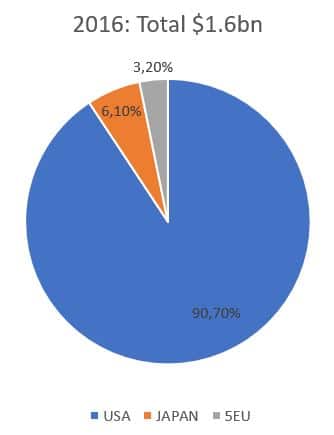
The prescription drug market for IBS was valued at $1.6 billion across the 7MM* in 2016 and experts expect it to increase to $2.6 billion in 2026. The US contributed over 90% of the market share in 2016, which is mainly linked to the large IBS population as well as high prescription drug prices in this market compared with the 5EU and Japan.
* 7MM : US, 5EU (France, Germany, Italy, Spain, UK), Japan
HOW IBS IS DEFINED?
IBS is a commonly diagnosed, complex, and widely encountered gastrointestinal (GI) condition characterized by chronic abdominal pain and altered bowel habits in the absence of any organic or biochemical abnormalities. The global prevalence of IBS, predominant in women, is 11.2%, with a wide range in prevalence from 1.1% to 45.0% depending on the country and the criteria used to define IBS. Individual symptoms of IBS have limited accuracy to formally establish a diagnosis of the disorder and it is therefore considered to be a symptom complex.
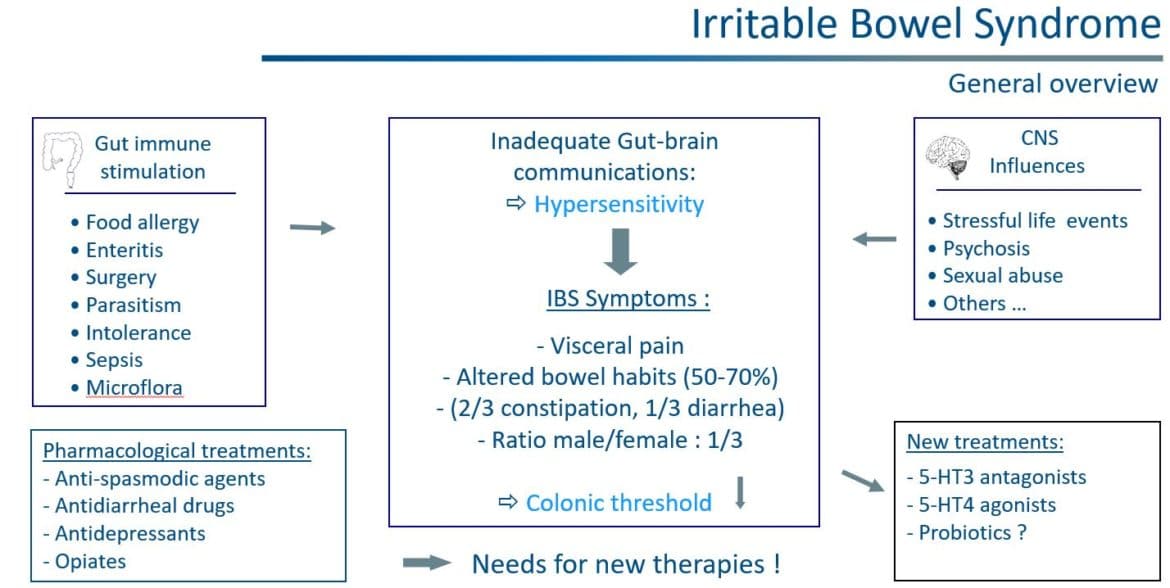
The etiology of IBS is complex and not well-understood. However, it is generally accepted that a combination of genetic and environmental factors plays a key role in the development of IBS. Other factors such as disturbances in GI motility, mucosal barrier disruption, dysbiosis of the gut microbiota, visceral hypersensitivity, food sensitivity, dysfunction of the gut-brain axis and stress response involving neurotransmitters are also believed to trigger the onset or exacerbation of IBS symptoms although their exact role remains unclarified. Finally, IBS has also a significant negative impact on a patient’s quality of life (QOL) and social functioning.
CURRENT TREATMENTS OF IBS: FROM DAILY HABITS TO MEDICINES
The initial steps in the management of IBS includes lifestyle and dietary modifications such as:
- improvements in physical activity
- relaxation time
- eating habits
- the use of probiotics
- the inclusion/exclusion of certain types of foods
- the use of fibers
Pharmacological treatment is recommended only if patients fail to respond to lifestyle and dietary changes, and is based on the patient’s subtype of IBS and the specific symptoms.
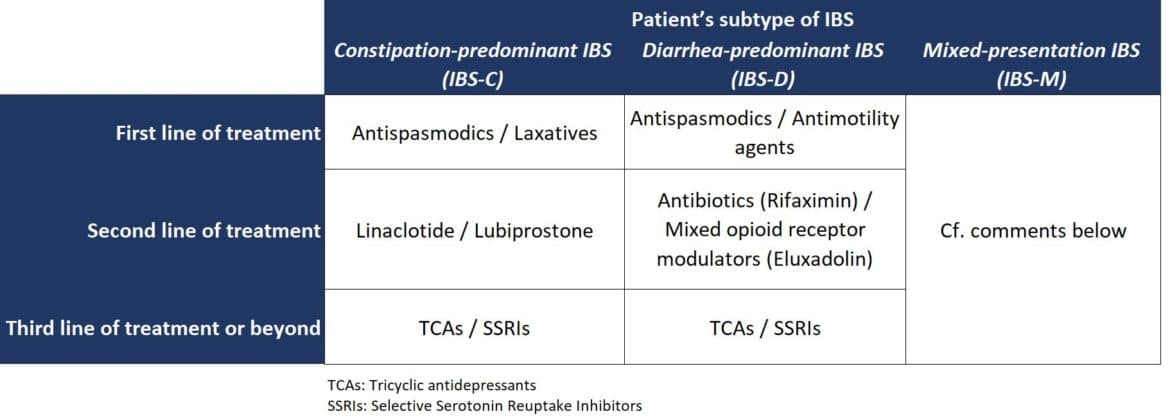
The treatment of patients with mixed-presentation IBS (IBS-M) is challenging. Physicians also treat these patients with the aforementioned pharmacotherapies according to the presenting symptoms at any given point in time. Experts claim the fact that although the presentation of symptoms in IBS-M patients is mixed (with alternating constipation and diarrhea), in general, these patients present predominantly with one of these symptoms. In such cases, physicians follow a treatment protocol that targets the predominant presenting symptom, rather than frequently alternating treatment with drugs for constipation and diarrhea.
In search of the perfect pain reliever

The IBS market is characterized by significant unmet needs.
The prescription drug market for IBS is underserved, as there are only six products currently approved and indicated for IBS in the 7MM. In many patients, prescribed drugs only show modest efficacy, are associated with negative safety profiles, or are used off-label with minimal direct clinical evidence, such as treatments for IBS-M.
The innovation would come to meet the following significant unmet needs for the treatment of IBS:
-
- Products with improved efficacy
- Products for the treatment of the IBS-D and IBS-M subtypes
- Products that effectively address IBS symptoms, such as abdominal pain and bloating
A field of research requiring a unique expertise
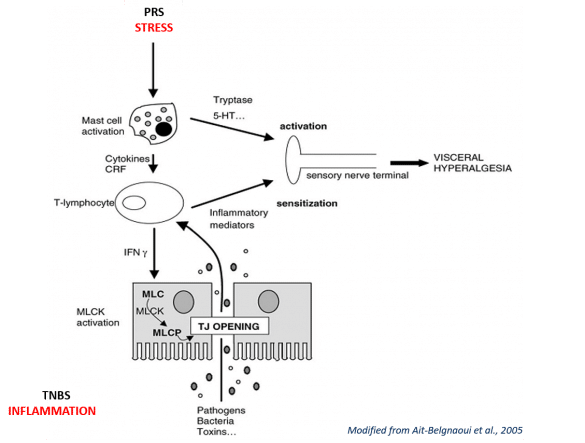
ANS Biotech offers two preclinical models of IBS from peripheral and central origin. Chemical irritants such as Trinitrobenzene sulfonic acid (TNBS) induce severe colonic inflammation and visceral hypersensitivity (peripherally mediated). Partial restraint stress (PRS), as a mild, non-painful, mainly psychological stressor, could trigger visceral hypersensitivity as well (centrally mediated). These models can be used to experimentally explore the pathophysiological aspects of this disorder. These assays are used to determine the potential antihypersensitive effects of compounds.
Advantages to collaborate with ANS Biotech
- High translatability to the clinic: pathophysiology and pain evaluation
- Most commonly used model for pain IBS
- Robust reproducibility
- Low inter-individual variability
To know more about ANS Biotech services, do not hesitate to contact us !
