Get your dual Analgesic/Safety fingerprint

ALGOGram™ 2.0 offers a dual focus on efficacy and safety; an invaluable advantage for researchers looking to understand both the therapeutic effects and potential adverse impacts of compounds in early-stage drug development.
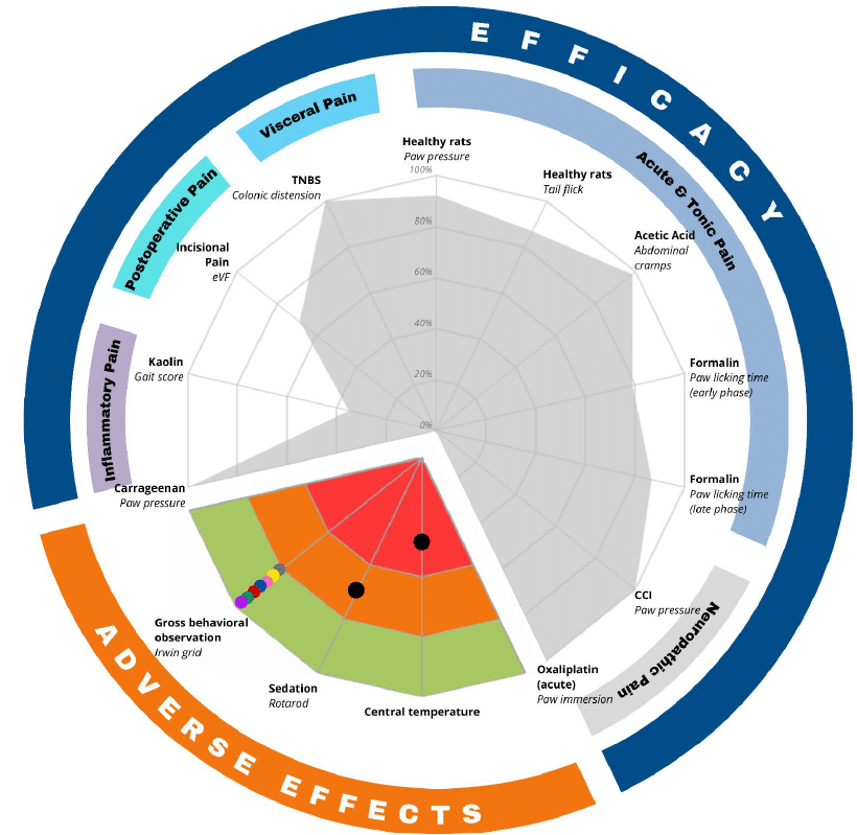
When profiling lead compounds in your discovery programs, you should consider ALGOGramTM 2.0, a unique in vivo screening tool that provides a clever method for simultaneously exploring analgesic efficacy across 10 fully validated pain models / tests in 5 different pain areas:
– Acute & tonic pain
– Inflammatory pain
– Neuropathic pain
– Postoperative pain
– Visceral pain
Safety assessment is integral to the drug development process, and ALGOGram™ 2.0 has been also designed to incorporate at early stage an evaluation of 3 safety parameters alongside efficacy testing:
– Central temperature measurement
– Irwin grid behavioral assessment
– Rotarod test for motor coordination
Combined together, ALGOGram™ 2.0 serves as a powerful “GO/NO GO” decision tool. This global fingerprint offers clear, actionable insights, minimizing the risk of progressing with ineffective compounds.
ONE STREAMLINED SOLUTION for both efficacy and adverse effect screening!
ENHANCE YOUR DRUG DEVELOPMENT
MINIMIZE THE RISK
SAFE AND PRECISE DESCISION






Link with in vitro data /
Drug repositioning
Dual Analgesic/Safety fingerprint
Crucial GO/NO GO Decisions
Leverage 18 years of data & AI to standardize Efficacy/Safety Profiles for New Compounds
13 assays seamlessly orchestrated during a 2 week experimental phase!
EFFICACY:
10 preclinical pain models and tests
5 pain areas
SAFETY:
3 assays
EXPERIMENTAL CONDITIONS:
Small group size (n=4)
Single time point
Single dose
Single administration
Reliance on historical database for vehicle
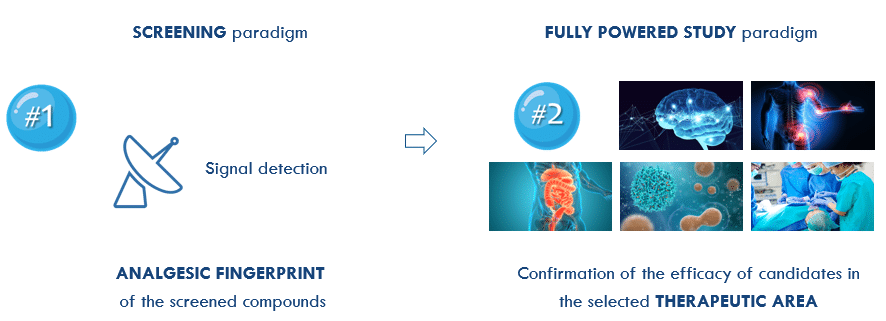
PROOF OF EFFICACY OF YOUR DRUG IN JUST 2 STEPS!
1- ALGOGram™ gives you the analgesic profile of your test articles by detecting efficacy in a given pain area, guiding you in your “GO / NO GO” decisions.
2- The analgesic fingerprint provided by ALGOGram™ can be confirmed and fine-tuned in fully powered studies as a follow-up to the initial screening step.