
We are thrilled to announce the launch of ALGOGram™ 2.0, the latest iteration of our innovative in vivo screening tool designed to advance pain research. Building on the solid foundation of ALGOGram™, version 2.0 not only continues its focus on the efficacy of analgesic compounds but also integrates comprehensive safety evaluations, providing researchers with a complete profile of their compounds.
What Makes ALGOGram™ 2.0 Different?
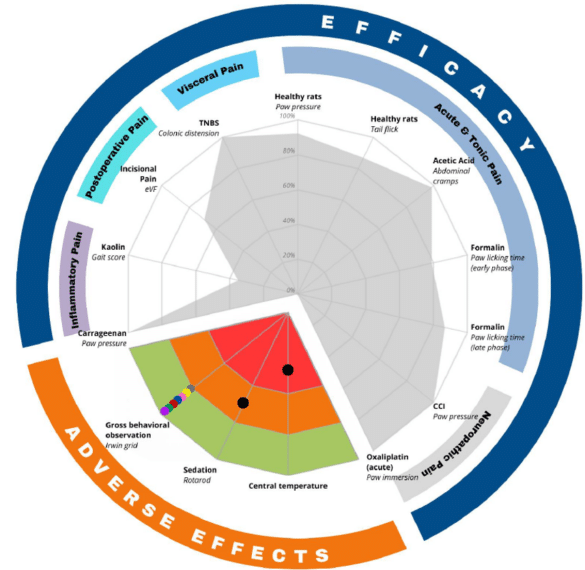
ALGOGram™ 2.0 focuses on both efficacy and safety with new integrated safety parameters critical to early-stage drug development:
- Central Temperature Measurement: Body temperature monitoring is essential for assessing the physiological effects of analgesics, ensuring subject health and stability throughout the study.
- Irwin Grid Behavioral Assessment: This comprehensive tool allows researchers to observe the behavioral effects of test compounds, identifying subtle side effects or adverse reactions that may not be immediately evident.
- Rotarod Test for Motor Coordination: The Rotarod test evaluates motor function and balance, helping researchers understand the impact of analgesics on motor abilities, a vital factor in assessing a drug’s safety profile.
Why Choose ALGOGram™ 2.0?
ALGOGram™ 2.0 offers a dual focus on efficacy and safety; an invaluable advantage for researchers looking to understand both the therapeutic effects and potential adverse impacts of compounds in early-stage drug development.
Efficacy: With 10 preclinical pain models covering five key pain areas, ALGOGram™ 2.0 provides a comprehensive analgesic profile of each test compound.
Safety: Safety assessment is integral to the drug development process, and ALGOGram™ 2.0 has been designed to incorporate at early stage an evaluation of 3 safety parameters alongside efficacy testing.
Combined together, ALGOGram™ 2.0 serves as a powerful “GO/NO GO” decision tool. This dual fingerprint offers clear, actionable insights, minimizing the risk of progressing with ineffective compounds.
Additionally, ALGOGram™ 2.0 enhances efficiency with streamlined processes. It operates with a small group size (n=4) per test, relying on single-dose administration at a single time point. These factors allow for faster data acquisition while reducing resource requirements, thanks to our extensive historical database that supports comparison and validation of test data.

Your go-to tool for informed decision-making!
👉 Interested in learning more?
Discover ALGOGram 2.0 and its capabilities on our website.
Stay ahead in pain research with ANS Biotech!
