
For this first blog, Laurent DIOP, Chief Scientific Officer at ANS Biotech, would like to focus on Neuropathic Pain and more specifically on Chemotherapy-induced peripheral neuropathy (CIPN). Since its creation in 2006, ANS Biotech is fully involved in this thematic and daily serves its Clients, all around the world, using relevant in vivo models for a unique goal, namely the relief of the patient’s suffering.
Predictions mention that at least 15-20% of patients are likely to suffer from NP during the course of the disease, and an even higher proportion at advanced stages.
Cancer-related pain can be caused by the cancer itself or by the cancer treatment and is often associated with insufficient prescribing of targeted analgesia.
In chemotherapy, the cumulative dose-limiting toxicity is a major problem due to the painful neuropathy induced by these antineoplastic agents. Among them, Oxaliplatin is a third-generation platinum-based chemotherapy drug widely used in the treatment of advanced metastatic colorectal cancer.
Chemotherapy-induced peripheral neuropathy (CIPN) is common and challenging complication arising from treatment with anticancer drugs. A number of factors have contributed to the increasing prevalence of CIPN: increased incidence of cancer, improved survival and cancer cure rates.
The severity of CIPN is the major clinical problem. Moreover, chemotherapy affects the quality of life of patients. All these facts have an impact on the efficacy of oncological treatment and the survival. The pharmacological treatment for CIPN is limited. Rodent models of CIPN-induced peripheral neuropathy have been developed using a range of dosing treatments to reproduce the clinical symptoms in the patients. The mechanisms of oxaliplatin-induced peripheral neuropathy are complex with changes in ion channels (sodium, potassium, and calcium), transient receptor potential channels, mitochondrial dysfunction, and immune cell interactions.
Oxaliplatin (OXA) is a first-line and adjuvant treatment for colorectal cancer, and also used in the treatment of esophageal, stomach, liver and pancreatic cancers. The severity of OXA-induced peripheral neuropathy requires a reduction in the dose of chemotherapy or even stopping of treatment before completing the planned course. During the first cure, 65-98% of patients develop acute transient OXA-induced peripheral neuropathy and the duration may last up to one week. After several cycles of chemotherapy, symptoms last up to 3 weeks or longer. Cold hypersensitivity is the major symptom of this peripheral neuropathy. It is often associated with paraesthesia, numbness, inability to detect temperature changes and loss of fine motor function. Some of risk factors are associated with an increased risk of developing OXA-induced peripheral neuropathy such as older age, medication, comorbid health status , high BMI, low serum albumin and use of opioids.
Mechanisms Of Oxaliplatin-Induced Peripheral Neuropathy
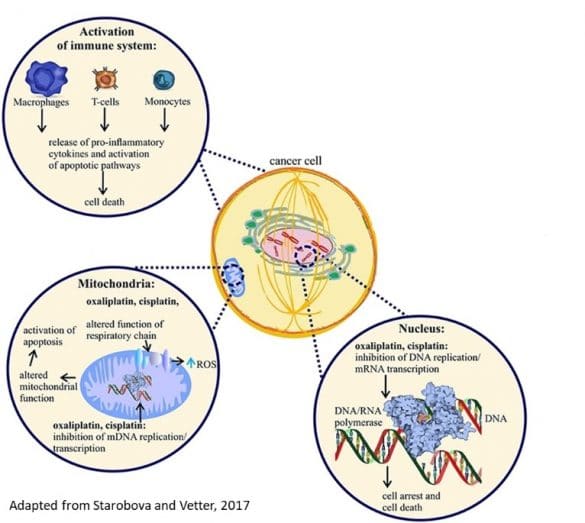
- The first mechanism of OXA is localized in nucleus of cancer cells. The platinum compounds block DNA replication and mRNA replication via DNA polymerase, blocking DNA replication/transcription and inducing apoptosis
- Second action is localized in mitochondria, oxaliplatin produces an alteration of respiratory chain and increases reactive oxygen species. In addition, oxaliplatin blocks also the replication of mitochondrial DNA. These mechanisms alter the mitochondrial function and induce activation of apoptosis phenomena.
- Third action of oxaliplatin is an activation of immune system via macrophage, t-cells and monocytes; the stimulation of these cells produces the release of pro-inflammatory cytokines. Oxaliplatin metabolite oxalate is very well known calcium chelator and leads to an increase of Na conductance.
In peripheral neurons, oxaliplatin blocks the replication of mitochondrial DNA and altered mitochondrial function. It produces an increase of reactive oxygen species and also activation of apoptotic pathways. All these mechanisms lead to altered excitability of peripheral neurons. Chemotherapy agents are well known to cause drastic immunosuppression. However, activation of the immune system participates to the destruction of tumors cells. This neuroinflammation contributes to the development of oxaliplatin-induced peripheral neuropathy. One example the efficacy of an antibiotics (tetracycline minocycline)( known to inhibit macrophages/monocytes and microglia on oxaliplatin-induced hyperalgesia . The activation of immune cells such as macrophages, T-cells and monocytes produces an increase of inflammatory cytokines such as IL-6, IL-8 IL1B and TNF-alpha. OXA produces alteration of immune cells, in particular, on calcium level. And, on microglia, oxaliplatin produces a release of TNF-alpha and produces attraction and activation of immune cells. Finally, these mechanisms produce activation of innate immune system and neuroinflammation.
In animal models, acute OXA models show a rapid onset of both cold and mechanical allodynia. Several studies have reported cold and heat hypersensitivity with allodynia and hyperalgesia associated with mechanical allodynia, remaining after a 3-week follow-up. These symptoms are analogous to the clinical problem where patients most frequently report sensitivity to cold mechanical stimuli soon after the first drug administration. In humans, an hyperexcitability of sensory neurons have been described. The sensitization of Ab- and C-fibers, but not Ad-fibers, contributes to the development of OXA -induced mechanical and cold allodynia in animals.
In vitro, oxaliplatin impairs sensory neurons arborization through up-regulation of neurite growth related molecules that decreases neurite length. An increase in substance P immunoreactivity has been observed in the superficial layers of the spinal dorsal horn. Several studies in humans and animals have demonstrated axon degeneration after long-term administration of platinum agents, including the loss of large myelinated, small unmyelinated, and intra-epidermal nerve fibers which may be connected to the development of sensory-motoric peripheral neuropathy. OXA alters sensory nerve conduction in animal models but also in humans. OXA damaged myelinated fibers of the sciatic nerve, diminished the action potential amplitude and reduced the nerve conduction velocity of the caudal sensory nerve. However, in neuropathic patients, the reduction in nerve conduction is not correlated with the symptoms. Integrity of the most distal parts of unmyelinated sensory axons can be determined through evaluation of skin punch biopsies. In rodent models, punch biopsies of plantar foot pads are stained with an axonal marker, PGP 9.5 antibody. After this treatment, there are significant loss of nerve fibers that innervate the epidermis. The unmyelinated fibers and terminal nerve arbors are the major sites of OXA-induced neurotoxicity.
Pharmacology In Oxaliplatin-Induced Peripheral Neuropathy

In the literature of the randomized controlled trials in OXA-induced peripheral neuropathy, a consensus for the treatment of these PN patients is not evident.
A moderate recommendation is for duloxetine. Indeed, duloxetine is effective on pain scores, numbness in OXA-treated patients compared to placebo-treated patients. Several pharmacological studies have shown that administration of duloxetine inhibited in dose-related manner OXA-induced cold and mechanical allodynia. Moreover, thermal place preference has shown that oxaliplatin-treated animals avoid cold temperatures.
For the other antidepressants, amitriptyline a tricyclic antidepressant shows any improvement of OXA-induced symptoms in clinical studies. In OXA models, one administration of amitriptyline had no effect on acute cold allodynia. The chronic treatment showed the reduction of cold hypersensitivity. In mice, milnacipran prevented acute OXA-induced cold allodynia, inhibited chronic oxaliplatin-induced mechanical allodynia. Venlafaxine reversed chronic oxaliplatin-induced cold allodynia in rats.
For anticonvulsant compounds, gabapentin is widely used in the treatment of peripheral neuropathy. There are several clinical studies with contradictory results: one pilot study showed reduction of CIPN symptoms and more large study indicated no effect of gabapentin. In preclinical studies, gabapentin is often used as a positive control to assess potential analgesics. Gabapentin prevents acute and chronic cold allodynia induce by OXA. For pregabalin, the literature is very controversial for the efficacy of pregabalin to treat OXA-induced symptoms. In animal models, pregabalin is effective in acute and chronic OXA-induced cold allodynia.
In patients with cold allodynia induced by OXA, the treatment with selective sigma1 antagonist showed the reduction of cold hypersensitivity. In animal models, the single administration of this compound produced in dose-related manner a reduction of oxaliplatin-induced cold allodynia. The chronic treatment of this sigma1 antagonist produced in dose-related manner a reduction of oxaliplatin-induced cold allodynia.
In conclusion, there are several pharmacological models to reproduce the different phases (acute and chronic) of development of cold allodynia and symptoms induced by OXA. These mechanisms are complex with changes in ion channels (sodium, potassium, and calcium), transient receptor potential channels, mitochondrial dysfunction, and immune cell interactions. On a positive note, there are many potential sites for modulation, with novel analgesic approaches. The strategy from bed to bench and from bench to bed to have translational models, will permit the discovery of new effective treatments on clinical symptoms.
“At ANS Biotech, translational pain research is one of our founding pillars, leading us to develop original and relevant preclinical models inspired by clinic. Since the creation of the company in 2006, we have developed an impressive experience in the preclinical pharmacology of Neuropathic Pain. Attentive to our clients’ needs, over the years, we propose a constant update of clinically relevant in vivo models in this therapeutic area.”


You may also be interested in this article dealing with neuropathic pain in a more general manner.
